INTERDISCIPLINARY SEMINAR REPORT
Prof Mohamed Mutocheluh
Leader: Infectious Agents and Cancer Research Group
Seminar date: 30th May 2024
Venue: Clinical Microbiology seminar room
Mode of delivery: Both in-person and online
A new antimalarial herbal Cryptolepis Sanguinolenta based preparation reduced drug-resistant HIV load in a series of clinical reports
Globally, about 39 million people were living with HIV as of 2022 according to UNAIDS/WHO 2023 report. Further, about 1% of adults (15-49 years) worldwide are living with HIV although the burden of the epidemic varies in different countries. Interestingly, the WHO Africa Region remains the most severely affected, accounts for more than two-thirds of people living with HIV worldwide. In Ghana, HIV prevalence is around 1.8% as of 2023 according to the Ghana Aids Commission.
UNAIDS/WHO reports suggest in 2022, about 28 million people, out of the estimated 39 million people living with HIV (PLHIV) were receiving antiretroviral therapy (ART) globally. It means about 29% of PLHIV have no access to ART. The widespread use of ART resulted in the emergence of HIV drug resistance (HIVDR) leading to a huge challenge in achieving optimal ART therapy outcomes particularly in Africa.
The UNAIDS/WHO and other international organizations set out an ambitious agenda 95-95-95 by 2030. It means 95% of infected persons know their status, 95% of those diagnosed receive treatment, 95% of people treated are virally suppressed to maintain undetectable status. The author thought that if about 29% of PLHIV currently have no access to ART and about 26% of PLHIV do not respond to first line therapy then the world may not achieve the agenda 95-95-95 and therefore alternative complementary medicine should be explored to bridge the gap.
In August 2017, Prof. Mohamed Mutocheluh and his group documented that cryptolepine an herbal compound activated and augmented the antiviral immune defense pathway in mouse macrophages (a type of blood cells). The group went on to publish how cryptolepine activated the antiviral immune defense system at the molecular level. Subsequently, the group translated their findings into clinical practice where it was shown a cryptolepis sanguinolenta based preparation inhibited hepatitis b virus (HBV) replication and cleared the viral load by 99% in a clinical case study. See the link to JoyNews Live – [https://www.youtube.com/watch?v=cNdlvMiZtf4&t=46s].
The Mutocheluh group later teamed up with clinicians and researchers at the Komfo Anokye Teaching Hospital to study the effect of the cryptolepis sanguinolenta preparation on a PLHIV who was not responding to second line ART.
Briefly, a 27-year-old HIV seropositive male on standard second-line antiretroviral therapy (ARV) of tenofovir, lamuvidine and dolutegravir continued to show increasing HIV load over a 36-week period (Fig 1). This pattern of sustained increase in HIV load is suggestive of the replication of drug-resistant strains. He volunteered and consented to participate the study in which he was administered the aqueous extract of Cryptolepis sanguinolenta (C.S) for a period of 10 weeks commencing 1st November 2022 to 13th January 2023, in addition to continuing with the standard ARV treatment. This treatment regimen coincided with a 40% reduction in HIV load following the aqueous extract of C.S administration.
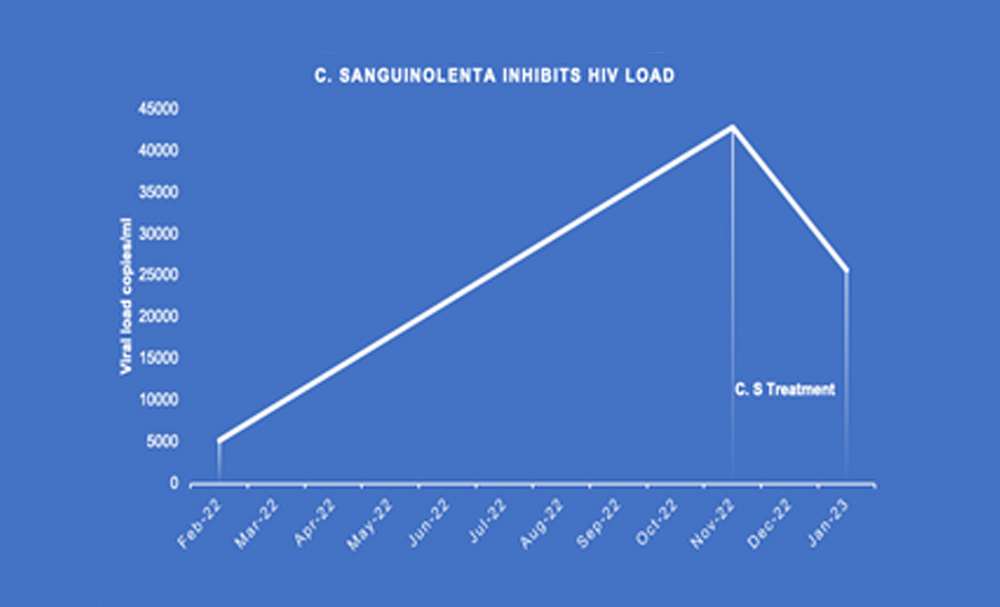
The patient took the aqueous extract of C. sanguinolenta from 1st November 2022 to 13th January 2023 (C. S Treatment). When the HIV viral load was measured, a 40% reduction was shown compared with pre-C.S administration. HIV-1 load was estimated with COBAS Ampliprep/CobasTaqMan HIV-1 Viral Load Assay.
The clinical case study participant did not report any adverse reaction and nor were abnormalities in haematological, liver and kidney functions identified before, during and after the aqueous extract of C.S administration.
The team plans to expand the current work to generate a proof-of-concept document to source external funds to start a clinical trial in the future. The aim is to unravel at the molecular level how C.S interact with the immune system to inhibit HIV replication.
Dr Robert Schwartz has extended a helping hand to the Dr Mutocheluh research group to offer laboratory space and technical expertise to execute the current proposal to its logical conclusion. Dr. Robert Schwartz is a Professor of Medicine at the Weill Medical College of Cornell University in New York City. Robert’s lab focuses on stem cell biology, tissue models, liver disease, infectious diseases, cancer genomics.